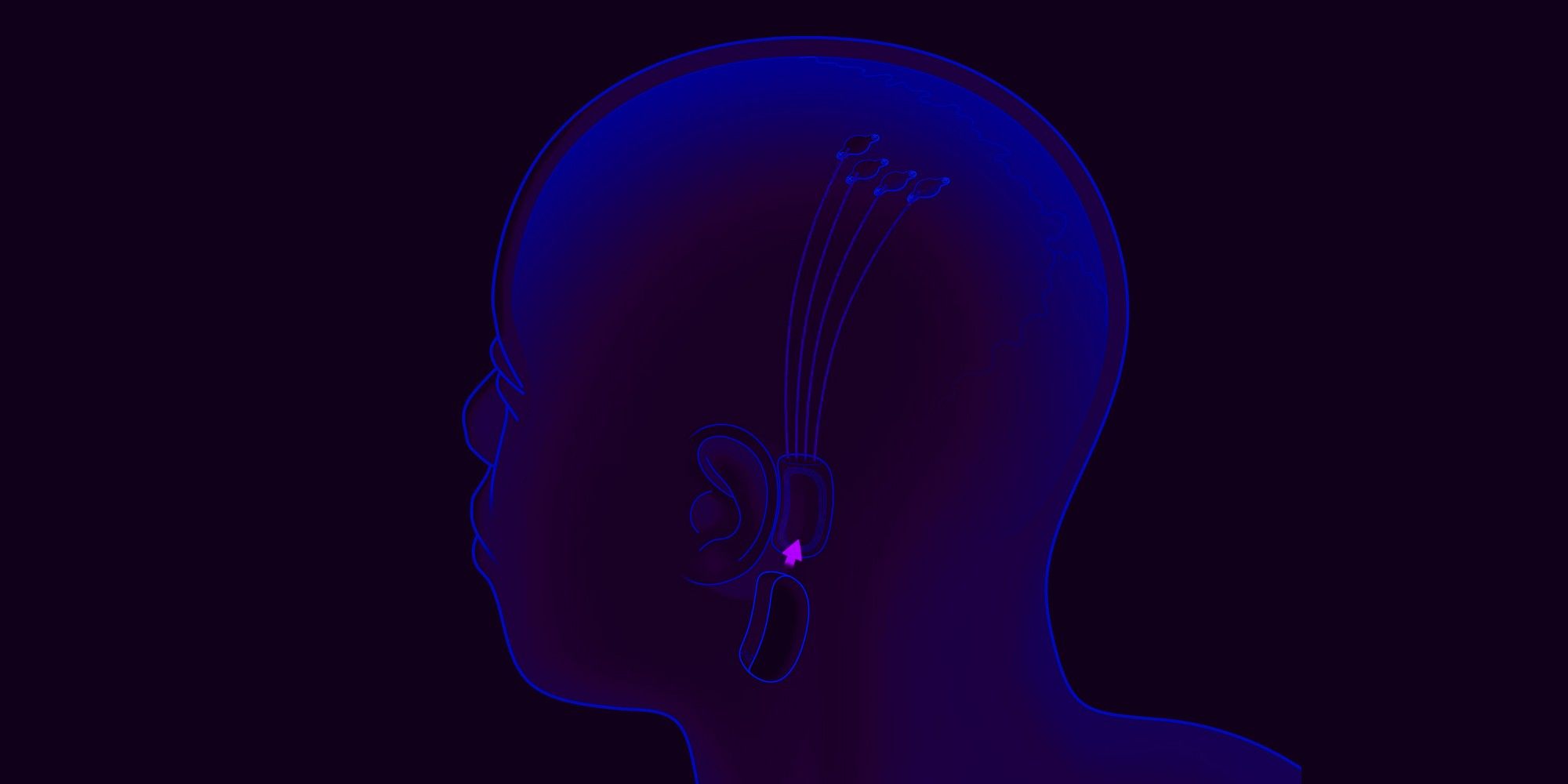
Elon Musk's secretive Neuralink company might finally be close to performing the first human trial of its brain-machine interface chip this year if a recent job listing is anything to go by. For the unaware, the company is making a device that can be surgically implanted at the back of a person's head, allowing them to interact with machines and perform tasks like controlling a phone or computer without any limb movement. One of the key objectives is to use the tech on people with paraplegia so that they can interact with the world via machines around them.
The company received $205 million in funding back in August last year to accelerate the tech's market arrival. In April of 2021, Neuralink showed a monkey playing pong wirelessly via two Neuralink interfaces, one implant on each side of the brain. In addition, going back a few months in February, Musk tweeted that the company was closely working with the United States Food and Drug Administration (FDA) to get the tech approved for safety. Musk noted that the company expected to begin human trials in 2021, but that didn't happen. It appears that the company is giving its dreams of a Neuralink human trial another push, with 2022 likely being the year it might finally happen.
As per a Neurlink job posting on Greenhouse boards, the company is looking for a clinical trial director in Fremont, Calif. "As the Clinical Trial Director, you'll work closely with some of the most innovative doctors and top engineers, as well as working with Neuralink's first Clinical Trial participants!" says the job listing. The clinical trial director will oversee the whole process of clinical trials ranging from training and monitoring to getting through the regulatory hassles and compliance duties. The job listing doesn't mention a launch timeline for Neuralink's human trials, but Musk told The Wall Street Journal that he hoped to put the brain implants in human subjects in 2022.
Is 2022 Finally The Year For Neuralink?
However, getting U.S. FDA approval is no cakewalk, as it's a multi-stage process. Following what is known as a feasibility test, a pivotal device examination is conducted in the wake of project submission and review. But at this point, it is unclear at what stage of FDA approval Neuralink is currently sitting. And given the ambitious scope of treatment Elon Musk has promised, the agency will likely take a long time to approve the advanced tech towards market readiness. "I think we have a chance with Neuralink to restore full-body functionality to someone who has a spinal cord injury. Neuralink's working well in monkeys, and we're actually doing just a lot of testing and just confirming that it's very safe and reliable and the Neuralink device can be removed safely," Musk said at The Wall Street Journal's CEO Council summit.
However, Musk's promises haven't always come to fruition, with long delays being a recurring theme. For example, in 2019, Musk claimed that human trials of Neuralink's brain-computer interface implants would begin in 2020, which didn't materialize. However, the FDA is not entirely averse to the tech. Late last year, it approved a similar tech from a rival New York-based startup called Synchron to test its brain-computer interface on human subjects as part of the agency's feasibility study. On the other hand, experts are concerned that hackers can compromise devices like Neuralink's implant to extract highly personal information such as thoughts, emotions, sexual orientation, or religious inclination, among others.
Sources: Greenhouse, The Wall Street Journal, CNBC