Apple's increasing use of magnets in its devices, such as for the MagSafe charging accessories, is raising some questions about their safety for use by those with pacemakers and other medical devices. Strong magnetic devices are known to have the potential to interfere with the normal function of medical devices or to activate special modes of operation. The US FDA recently released a statement about consumer electronics in this regard.
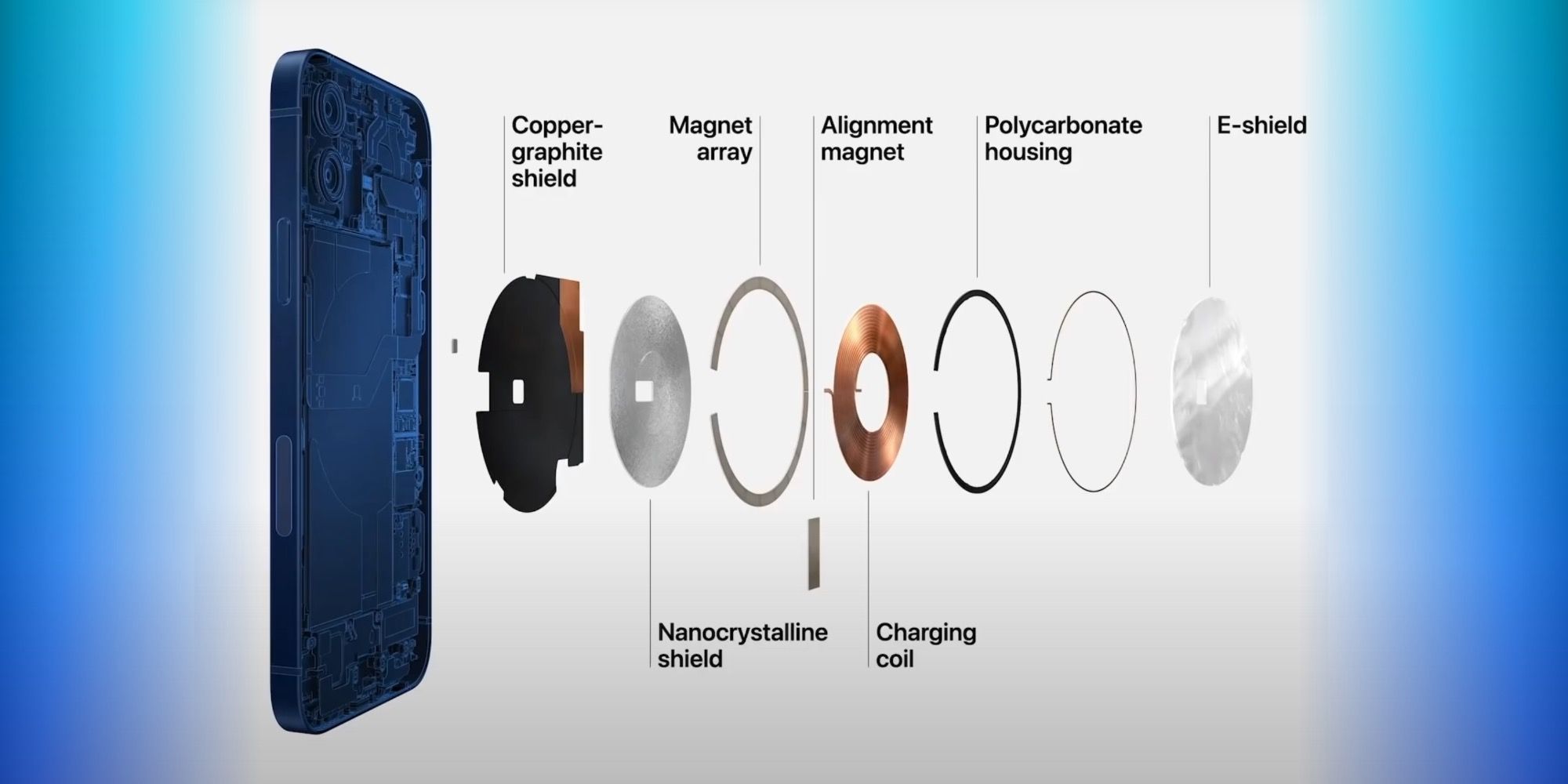
Apple sells many devices and accessories that incorporate strong magnets and has done for years. The original MagSafe charger for the 2006 MacBook Pro used magnetic attachment to prevent damage to the laptop if the charging cable was tripped over or pulled accidentally. The second-generation iPad, which was released in 2011, included magnets that allowed the device to sleep when a magnetic cover was placed over the screen and wake when removed. The 2018 iPad Pro can be suspended from magnets inside the Magic Keyboard, allowing it to hover about an inch above a desk. And the iPhone 12 has MagSafe with magnets strong enough to hold lightweight accessories to the back of the device.
Now, the US Food & Drug Administration (FDA) has issued a cautionary statement about consumer electronics that contain high-field-strength magnets, warning of the potential to affect the operation of implanted medical devices, including pacemakers and cardiac defibrillators. While the government organization did not mention any brand names, Apple, which introduced MagSafe charging with the launch of the iPhone 12, is among the most prominent manufacturers to incorporate strong magnets in its devices. Mobile phones have been identified as a potential risk for many years, so this is not a new phenomenon and medical professionals have already been aware of the need to use caution. The FDA stated that the current belief is that the risk is low, but expects the use of magnets to increase and therefore was advising those with questions to ask their health care provider.
How An iPhone Can Affect A Pacemaker
The FDA message explained that many implanted medical devices are designed to switch to a different mode of operation when a magnet is placed nearby. This 'magnet mode' allows medical professionals to perform certain procedures, such as Magnetic Resonance Imaging (MRI) without causing harm to the patient or damage to a pacemaker or other device. As these are implanted inside the body, a strong magnet allows quick and easy device interaction without the need for surgery to change modes. For its part, Apple advises keeping the iPhone 12 and MagSafe accessories more than six inches away from implanted medical devices and more than a foot away when the iPhone 12 is charging.
As 'magnet mode' is an intentional design of medical devices, triggering this mode would be unlikely to cause immediate failure or damage to the device, but rather would place it into a mode that prevents interference with medical procedures. For example, a pacemaker might switch to asynchronous operation rather than adjusting to cardiac events and a cardiac defibrillator may temporarily suspend tachyarrhythmia detection. Removing the external magnet from the vicinity of the medical device should restore normal operation, but this is intended to be done under medical supervision. For those with implanted medical devices, there is no need to fear using an iPhone 12, MagSafe accessories, or other electronic devices that incorporate magnets — as the FDA states, the risk of interaction is low — but awareness is important and can help to prevent any accidents.